Pipeline
Pipeline Overview
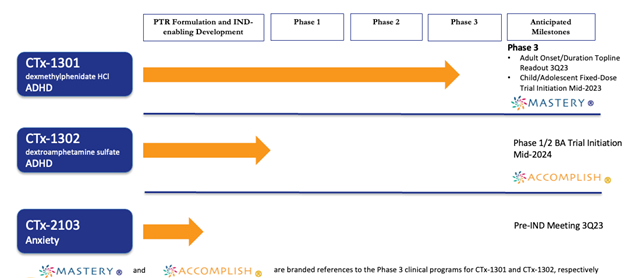
Cingulate® completed a proof-of-concept trial in human subjects to validate its Precision Timed Release™ (PTR™) drug delivery platform and in June 2019, announced positive results from a Phase 1/2 study of CTx-1301 (dexmethylphenidate) in ADHD patients establishing safety, tolerability, comparative bioavailability, and dose proportionality versus Focalin® XR.
Cingulate currently has three (3) product candidates, CTx-1301 (dexmethylphenidate), CTx-1302 (dextroamphetamine) and CTx-2103 (anxiety) in the pipeline. All three therapies utilize Cingulate’s innovative Precision Timed Release™ (PTR™) drug delivery platform technology. The two initial candidates are designed to fulfill long-standing unmet needs of patients with ADHD. No known competitive product currently in development is poised to overcome all unmet needs:
- Entire active-day duration and fast onset of action
- Elimination of need for a ‘booster/recovery’ dose of short-acting stimulant medication
- Improved tolerability including minimization or elimination of rebound/crash symptoms associated with early medication 'wear-off'
Cingulate plans to initiate a Phase 3 adult dose-optimization study to assess the onset and duration of efficacy and safety of CTx-1301 in adults with ADHD. The study is expected to commence in December 2022. Cingulate expects to begin the CTx-1301 Phase 3 fixed-dose pediatric and adolescent safety and efficacy study in mid-2023
Cingulate® also intends to utilize its PTR™ technology to expand and augment its clinical-stage pipeline by identifying and developing additional product candidates in other therapeutic areas where two or more active pharmaceutical ingredients (API) need to be delivered several times a day at specific, predefined time intervals and released in a manner that would offer significant improvement over existing therapies. Potential future candidates are being evaluated.
Cingulate® has compiled a human formulation trial in human subjects for our third candidate, CTx-2103, to treat anxiety disorders in late 2022. CTx-2103 contains one of the most widely prescribed anxiolytic agents which must be taken several times a day. In 2020, United States sales for this API accounted for over $2 billion in the $5.2 billion anxiety market. CTx-2103 will be designed as a once-daily, multi-release tablet with clear differentiation and compelling advantages over standard treatment options.
- Allowing ADHD patients to pay for one medication versus two
- Allowing payers to reimburse for one ADHD medication versus two
- CTx-2103 (buspirone hydrochloride) is being developed for the treatment of anxiety. The once daily patient focused development product is currently under investigation.
Erosion Barrier Layer (EBL) formulation OralogiK ™ is licensed from BDD Pharma.

